The Problem:
Current technology for methods of forming catalysts on conductive or semiconductive substrates utilizes precious metals such as platinum to catalyze the reaction. This is a very expensive way to complete the reaction. Additionally, current methods are highly energy intensive, which is an undesirable trait.
The Solution:
The technology displays a MoX2 catalytic electrode formation method which affects alternative energy creation such as hydrogen from water by solar energy. Types of Moly Sulfides with good solubility are utilized to form a catalytic molybdenum sulfide (MoSx) coating on a substrate by kinds of coating methods (e.g., spincoating and low-temperature chemical vapor deposition). The MoSx layer formed in a way that shows great prospects as both a protection layer and an electrocatalyst for hydrogen evolution reaction (HER) due to its excellent stability and high electrocatalytic activity.
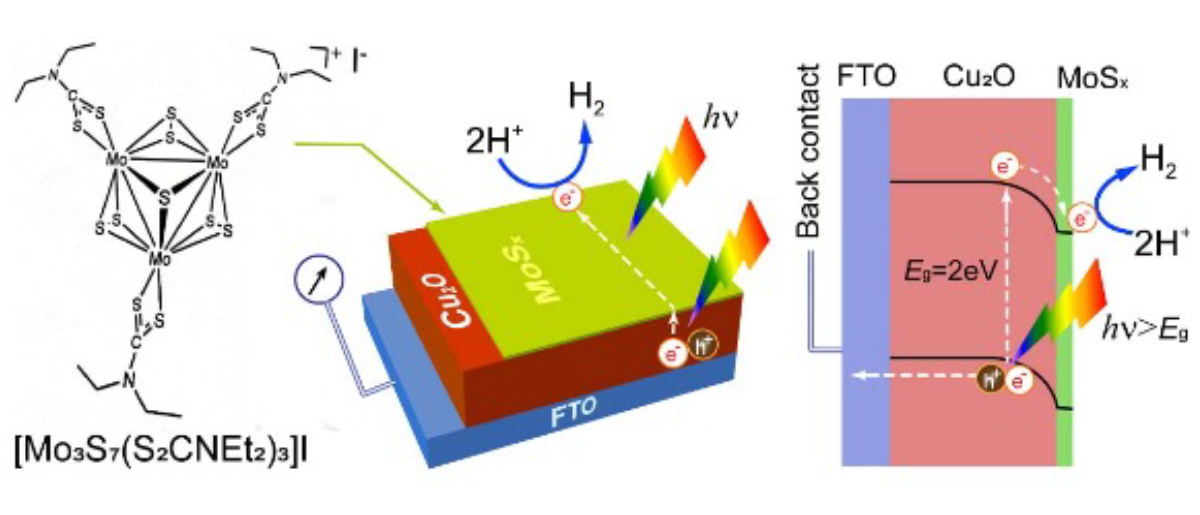 Schematic of a synthesis strategy to prepare surface-passivated Cu2O photocathode (MoSx/Cu2O/FTO) using MoSx (molecular structure of MoSx precursor is shown) as a bifunctional material to protect Cu2O and perform HER catalysis for solar water splitting application.
Schematic of a synthesis strategy to prepare surface-passivated Cu2O photocathode (MoSx/Cu2O/FTO) using MoSx (molecular structure of MoSx precursor is shown) as a bifunctional material to protect Cu2O and perform HER catalysis for solar water splitting application.
Benefits:
• Spray coating can be directly applied to the substrate.
• East control over film thickness.
• More-cost-effective than current methods.
• Potential for solar power.
The University of Alabama Office for Innovation and Commercialization(OIC) is a non-profit corporation that is responsible for commercializing University of Alabama technologies and for supporting University research. At OIC, we seek parties that are interested in learning more about our technologies and commercialization opportunities, and we welcome any inquiries you may have.